
Now let’s move to the molecular geometry of nitrous oxide! Note that, other two structures didn’t have their atoms with the lowest possible formal charge. Thus structure 3 is the final lewis structure of nitrous oxide.Īs mentioned in the rules, we can see all the atoms in the final lewis structure have their lowest formal charge. So in structure 2, one of the lone pairs of the side nitrogen is turned into a bonding pair of the middle nitrogen.īut still, there is a lack of 2 electrons, for which another lone pair is converted into a bond pair. Here, the octet of the middle nitrogen is not fulfilled.
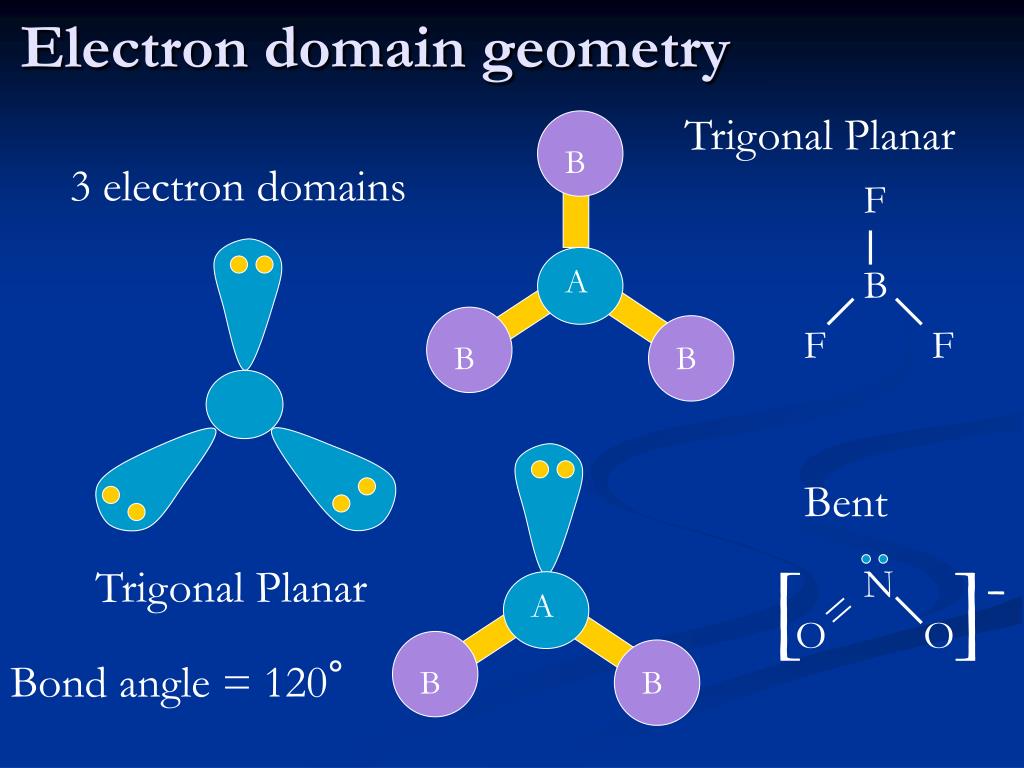
We can see after drawing the sketch, remaining electrons are given around the atoms surrounding ( Structure 1).

The following image attached can explain this more clearly, So one of the nitrogen is the middle atom.Īfter this, we need to draw the sketch of the molecule with only single bonds. In this case, nitrogen with the highest bonding sites is the central atom. Next, we need to decide on the central atom. Now let’s find out the lewis structure of N2O, You can calculate the same with the formula given below:. At last, make sure all the atoms are having their lowest possible formal charge.Give multiple bonds if required for fulfilling the octet of the atoms.Keep in mind to start with the electronegative atoms and proceed to the electropositive one. Fillup the octet of the atoms with the remaining electrons.Draw a skeletal structure with single bonds only.Choose a central atom generally the atom with the highest bonding sites.Do take care of +, – signs while calculating. Calculate the total number of valence electrons in the molecule.Have a look at the steps jotted down below:. There is a common way by which we can draw the lewis structure of any compound.
Electron domain geometry of so2 how to#
How to draw a lewis structureĪ lewis structure helps us to find out about the structure of the compound, types, and the number of bonds, physical properties, and how the compound interacts with other compounds.ĭrawing a lewis structure is pretty simple! So let’s move on to these parts one by one in detail.īefore going into the lewis structure of nitrous oxide, it’s better to know the steps to draw a lewis structure.

Now to understand every other reaction involving N2O, we need to know about its lewis structure, hybridization, and bonding. Moreover, nitrification and denitrification are two biological or natural processes that can produce nitrous oxide. Along with these, nitrous oxide is also a major component of the earth’s atmosphere.
There are many more reactions that are used for the preparation of N2O. This process is known as the Ostwald process. Ostwald process:- Oxidation of ammonia with manganese dioxide and with bismuth oxide as catalyst gives us nitrous oxide. Heating the mixture of sodium nitrate and ammonium sulfate gives us N2OĢ NaNO3 + (NH4)2SO4 -> Na2SO4 + 2N2O + 4 H2OĪlso, nitrous oxide can be formed by reacting urea, nitric acid, and sulfuric acid,Ģ (NH2)2CO + 2 HNO3 + H2SO4 → 2 N2O + 2 CO2 + (NH4)2SO4 + 2H2O Laboratory methods:- Preparation of nitrous oxide can be done in the lab as well. Industrial methods:- On an industrial scale, heating of ammonium nitrate gives us nitrous oxide and water vapor. Nitrous oxide can be prepared in more than one way.


 0 kommentar(er)
0 kommentar(er)
